Creation of micro and mesoporous Fe(III) materials utilizing organic template followed by carboxylates exchange for the low concentrations of arsenite removal |
Yasuo Izumi, Dilshad Masih, Ken-ichi Aika, and Yoshimi Seida, Microporous and Mesoporous Materials, 94(1-3), 243 - 253 (2006). [The PDF file]
Erratum [The PDF file]
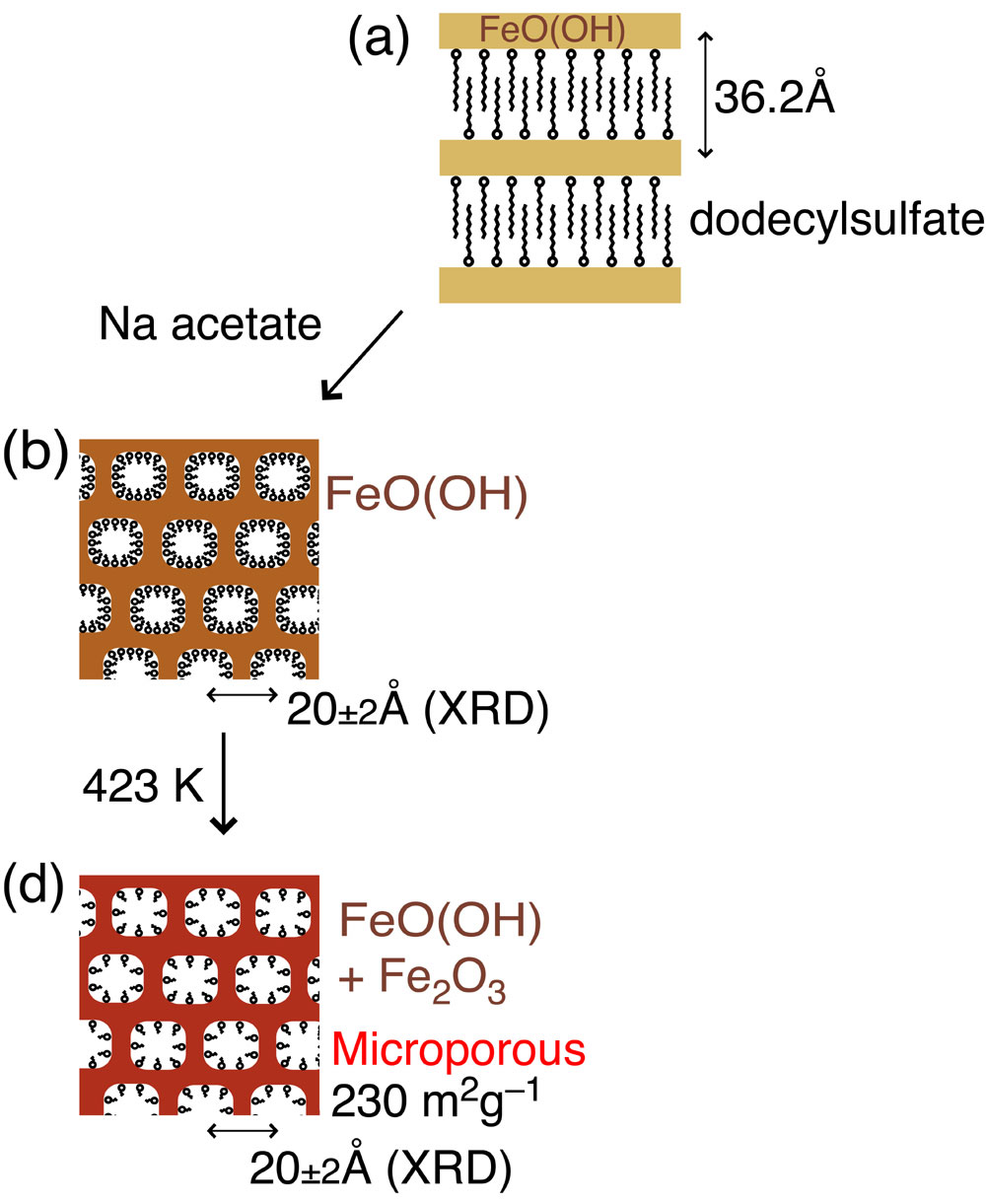
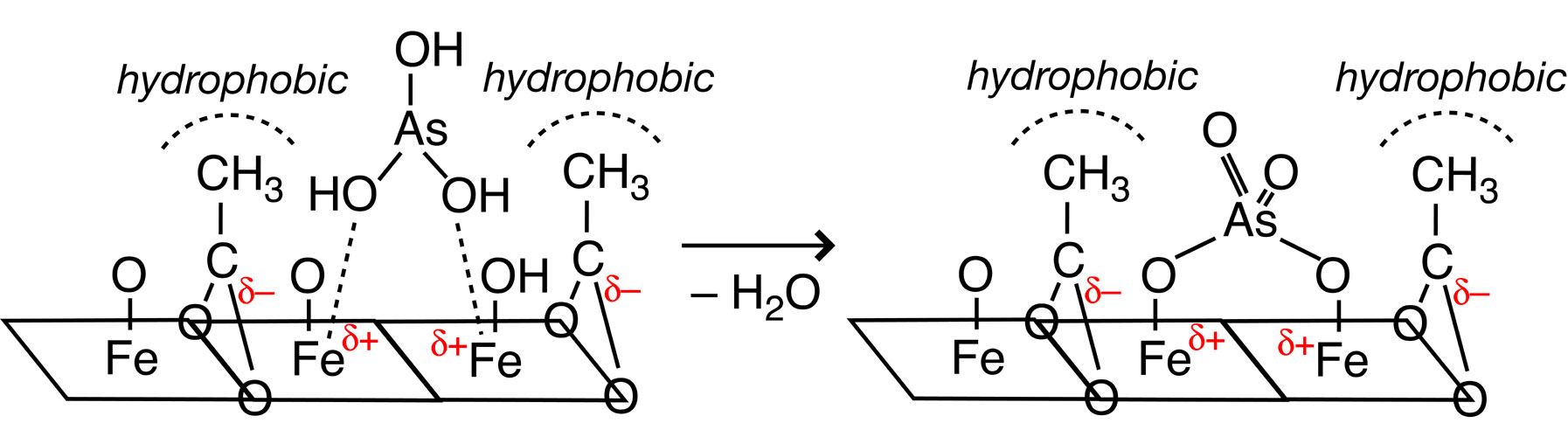
The instability of inorganic framework has been the greatest barrier to synthesize micro and mesoporous Fe(III/II) materials. This paper uses dodecylsulfate to organize lamellar-structure FeOx(OH)y composite followed by smaller carboxylates exchange (formate, acetate, or propionate) to create micropores. XRD, nitrogen sorption/desorption, HR-TEM, FT-IR, ICP, EPMA, TG-DTA, and Fe K-edge EXAFS measurements were used for the characterizations. The lamellar structure reorganized to wormhole-like framework stabilized with carboxylate anions adsorbed inside micropores. Upon heating at 423 K, a half of acetates/propionates diminished and the specific surface area increased to as much as 230 m^2g^-1. Based on the Fe K-edge EXAFS for FeOx(OH)y composite and derivative porous Fe(III) materials, Fe-O bonds were observed at 2.04 - 2.09 angstrom with the coordination number 5 - 6. Farther Fe„„„Fe bonds also appeared at 3.21 - 3.25 angstrom. The coordination number was obtained to 2 - 3, reflecting higher dispersion and higher surface area for these porous FeIII materials. The acetate-exchanged FeOx(OH)y heated at 423 K exhibited greatest saturated sorption amount (21 mg(As)g(adsorbent)^-1) and equilibrium sorption constant (1.0~10^7 ml g(As)^-1) in 0.2 - 32 ppm of arsenite test solutions among other relevant Fe(III) materials and Fe(III) nanoparticles intercalated between clay layers.
 |
 |
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group