|
Solar Cell with Photocatalyst Layers on Both the Anode and Cathode Providing an Electromotive Force of Two Volts per Cell |
Kazuki Urushidate, Shigemitsu Matsuzawa, Keisuke Nakatani, Jifu Li, Takashi Kojima, and Yasuo Izumi
ACS Sustainable Chemistry & Engineering ACS Sustainable Chemistry & Engineering, 6(9), 11892–11903 (2018). DOI: 10.1021/acssuschemeng.8b02166![]() [The
PDF file]
[The
PDF file]
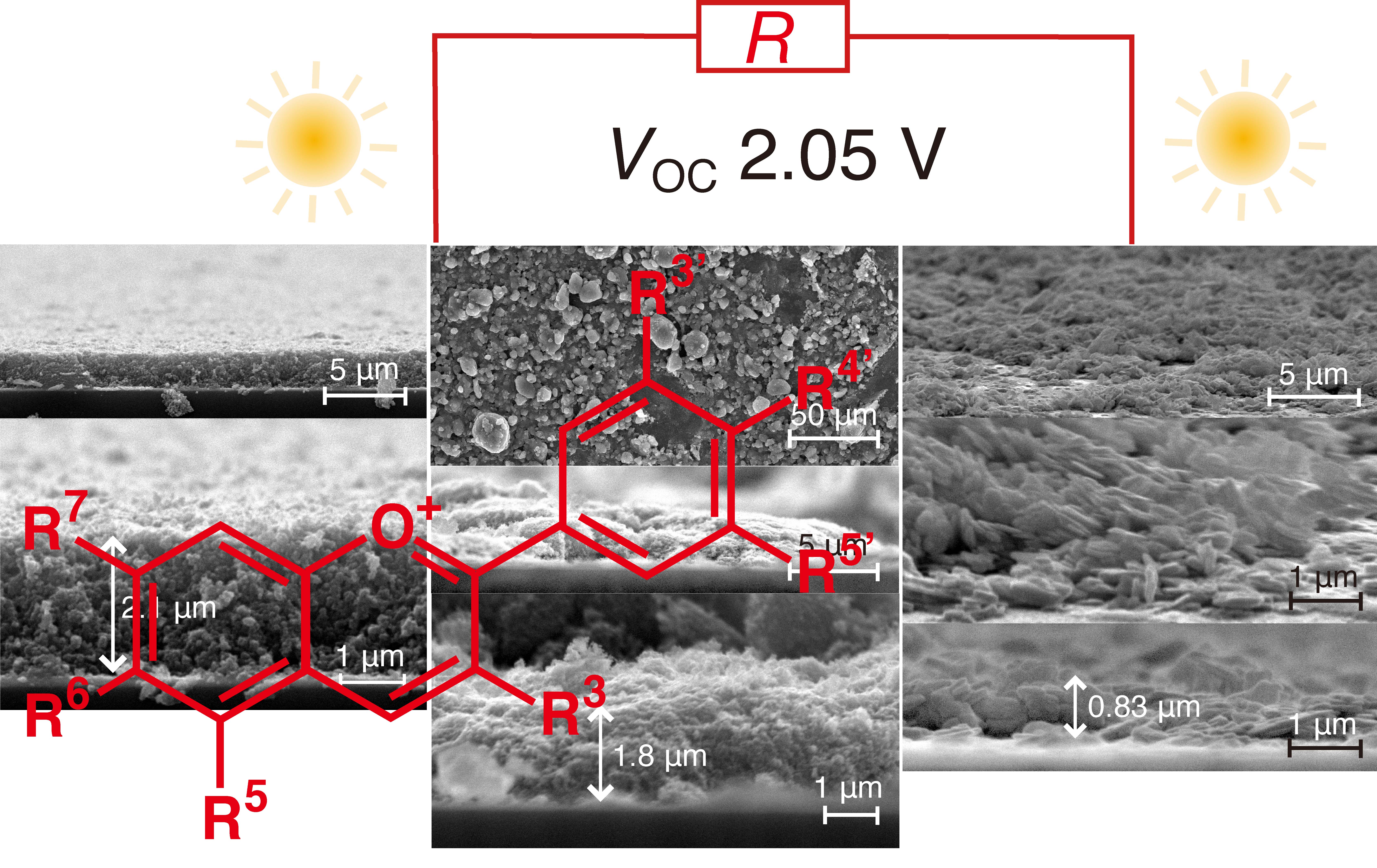
Solar cells and fuel cells are essential devices that convert sustainable energy into electricity. However, the electromotive forces they provide are in principle limited to less than 1 V, and consequently they need to be connected in series for practical use. In this study, photocatalyst layers with thicknesses of 0.8–2.1 μm were applied to both electrodes. The primary particle size of TiO2 was optimized (15–21 nm) and its performance was further improved by doping with organic dyes, e.g., anthocyanins. The electron conductivity of TiO2 was found to be a primary factor in the performance of the cells, but the film flatness also reduced resistance and improved the cell performance. Interestingly, the efficiency of TiO2 could be evaluated based on the exchangeable surface O atoms via its 18O2 exchange tests to suppress the reverse reaction step in water photooxidation, which occurs on the anode. UV and visible light were absorbed by TiO2 and the organic dyes, respectively, creating an electron flow path from the valence band to the conduction band of TiO2, then the HOMO to the LUMO of the organic dyes, then the anode, and finally the cathode via the external circuit. The flow of electrons and holes (separated from electrons in BiOCl on the cathode by UV–visible light) enabled a VOC value of 2.11 V and maximum power of 73.1 μW cm−2.
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group