|
Is water more reactive than H2 in photocatalytic CO2 conversion into fuels using semiconductor catalysts under high reaction pressures? |
Hongwei Zhang, Shogo Kawamura, Masayuki Tamba, Takashi Kojima, Mao Yoshiba, and Yasuo Izumi
Journal of Catalysis 352, 452-465 (2017).![]() [The
PDF file]
[The
PDF file]
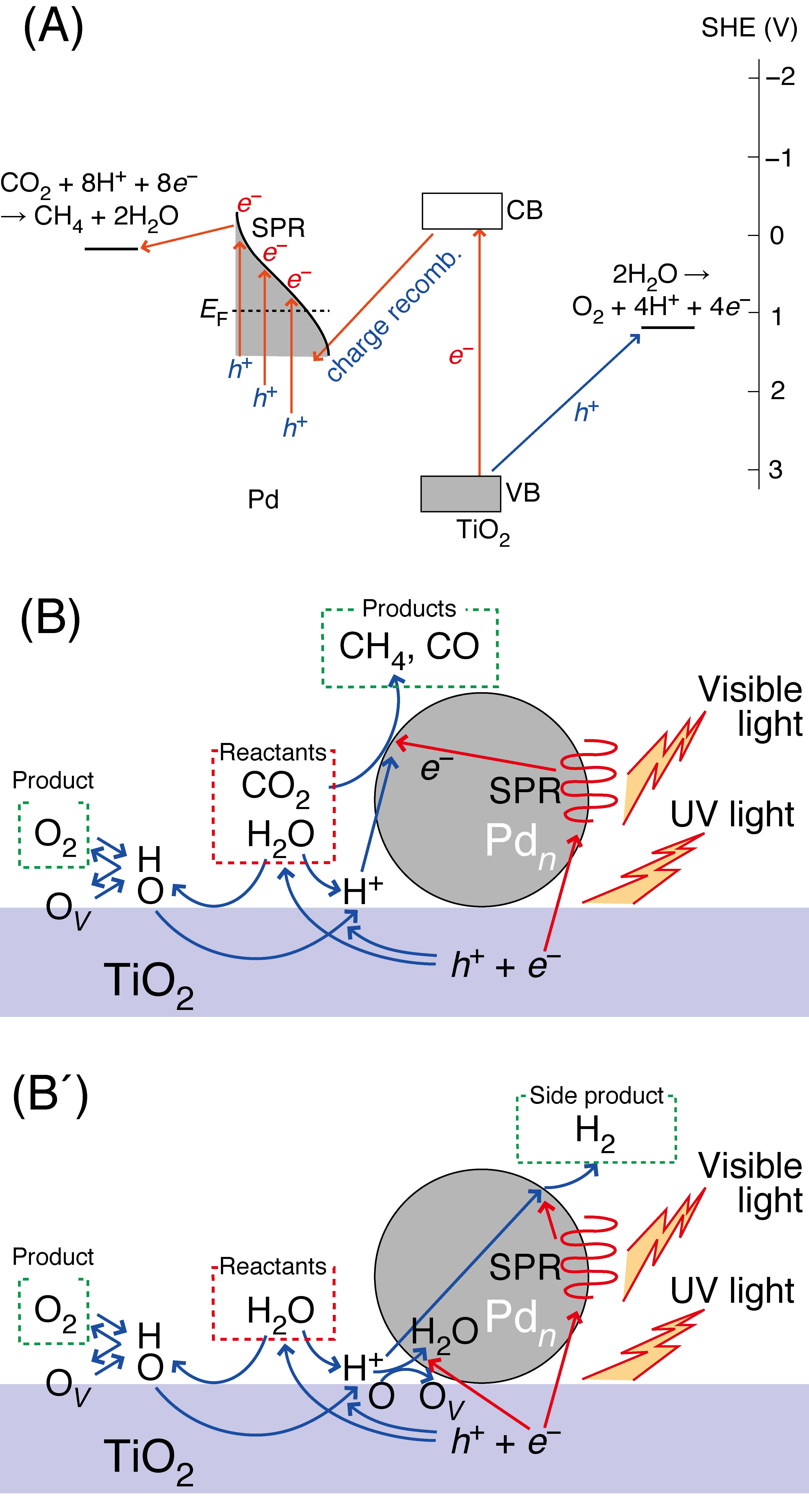
The reaction pressure in the photocatalytic conversion of CO2 into fuels is optimized between 0–0.80 MPa under CO2 and moisture. The higher reactivity of water than H2 was observed at higher pressure and the reason was clarified using several Å`10-μm-thick semiconductor-based photocatalysts. The best Pd/TiO2 photocatalyst produces methane with a reaction order of 0.39. The sum of independent total formation rates of C-containing compounds under UV and visible light does not account for that under UV–visible light, demonstrating synergetic reaction mechanism on Pd for CO2 reduction by excited electrons via surface plasmon resonance and on TiO2 for water oxidation. Active metallic Pd and O vacancy sites due to O2 formation from H2O are confirmed by in situ monitoring of EXAFS [N(Pd–Pd) = 5.9–6.2; N(Ti–O) = 5.2–3.5] and the decrease of the H-bound and bi/tri-coordinated OH peaks in FTIR. Effective redox-site separation explains the higher reactivity of water than H2.
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group