|
Photocatalytic conversion of carbon dioxide into methanol in reverse fuel cells with tungsten oxide and layered double hydroxide photocatalysts for solar fuel generation
|
Motoharu Morikawa, Yuta Ogura, Naveed Ahmed, Shogo Kawamura, Gaku Mikami, Seiji Okamoto, and Yasuo Izumi,
Catalysis Science and Technology, 4(6), 1644-1651 (2014). DOI: 10.1039/C3CY00959A[The PDF file]
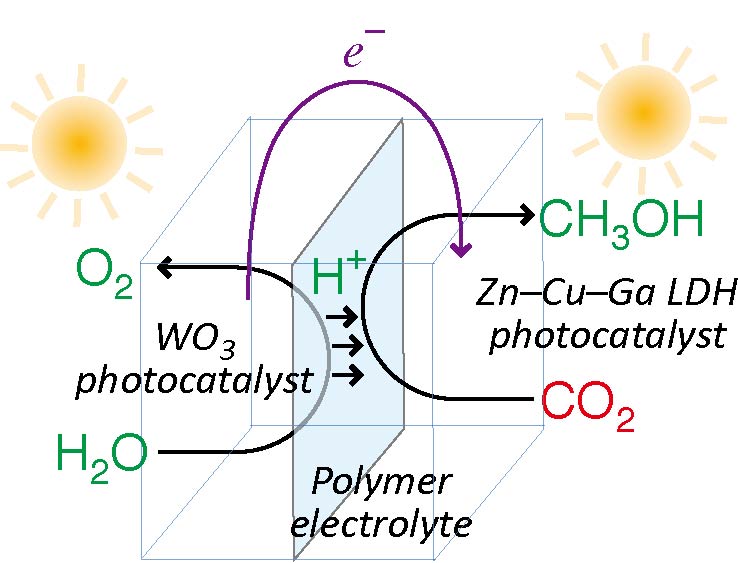
The phenomena of photocatalytic oxidation of water and photocatalytic reduction of CO2 were combined using reverse photofuel cells, in which the two photocatalysts, WO3 and layered double hydroxide (LDH), were separated by a polymer electrolyte (PE) film. WO3 was used for the photooxidation of water, whereas LDH, comprising Zn, Cu, and Ga, was used for the photoreduction of CO2. For this process, photocatalysts pressed on both sides of the PE film were irradiated by UV–visible light through quartz windows and through the space in carbon electrode plates and a water-repellent carbon paper for both gas flow and light transmission. 45% of the photocatalyst area was irradiated through the windows. The protons and electrons, which were formed on WO3 under the flow of helium and moisture, transferred to LDH via PE and external circuit, respectively. Methanol was the major product on LDH under the flow of CO2 and helium. The observed photoreduction rates of CO2 to methanol accounted for 68%–100% of photocurrents. This supports the effectiveness of the combined photooxidation and photoreduction mechanism as a viable strategy to selectively produce methanol. In addition, we tested reverse photofuel cell-2, which consisted of WO3 film pressed on C paper and LDH film pressed on Cu foil. The photoelectrodes were immersed in acidic solutions of pH 4, with the PE film distinguishing the two compartments. Both the photoelectrodes were completely irradiated by UV–visible light through the quartz windows. Consequently, the photocurrent from LDH under CO2 flow to WO3 under N2 flow was increased by 2.4–3.4 times in comparison to photofuel cell-1 tested under similar condition. However, major product from LDH was H2 rather than methanol using photofuel cell-2. The photogenerated electrons in the irradiated area of photocatalysts were obliged to diffuse laterally to the unirradiated area of photocatalysts in contact to C papers in photofuel cell-1. This lateral diffusion reduced the photocatalytic conversion rates of CO2, despite the advantages of photofuel cell-1 in terms of selective formation and easy separation of gas-phase methanol.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group