|
Photoconversion of Carbon Dioxide in Zinc–Copper–Gallium Layered Double Hydroxides: the Kinetics to Hydrogen Carbonate and Further to CO/Methanol |
Motoharu Morikawa, Naveed Ahmed, Yusuke Yoshida, Yasuo Izumi, Applied Catalysis B, 144, 561 – 569 (2014); DOI: 10.1016/j.apcatb.2013.07.065[The
PDF file]
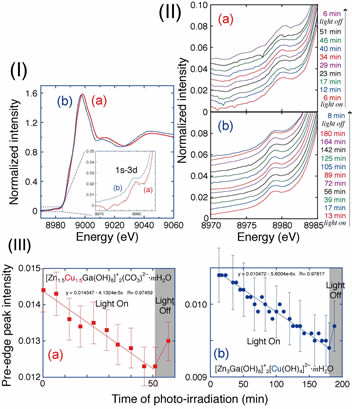
Photocatalytic reaction mechanism for the conversion of CO2 into methanol and CO using layered double hydroxides (LDHs) consisting of Zn, Cu, and Ga was investigated. X-ray absorption fine structure was applied to determine the LDH site structures and to monitor the diffusion of photogenerated electrons to CuII sites. Electron diffusion to Cu sites was an order of magnitude faster in the direction of the cationic layers (580 micromol h−1 gcat−1) than in the perpendicular direction. According to Fourier-transform infrared spectroscopy, CO2 was in equilibrium with hydrogen carbonate (1629 cm−1 for H13CO3) for the reaction with hydroxy group from the cationic layer or interlayer site and/or with interlayer water. The equilibrium reactions were faster for [Zn3Ga(OH)8]+2[Cu(OH)4]2–·mH2O (400–110 miromol h−1 gcat−1) than for [Zn1.5Cu1.5Ga(OH)8]+2(CO3)2–·mH2O. Furthermore, the reductive decomposition of hydrogen carbonate was suggested in H2 under UV-visible light, suggesting photocatalytic pathway to methanol/CO.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group