| Photocatalytic conversion of carbon dioxide into methanol using zinc-copper-M(III) (M = aluminum, gallium) layered double hydroxides |
Naveed Ahmed, Yoshiyuki Shibata, Tatsuo Taniguchi, and Yasuo Izumi,
Journal of Catalysis, 279(1), 123 – 135 (2011)![]() [The
PDF file][Supp.Info]
[The
PDF file][Supp.Info]
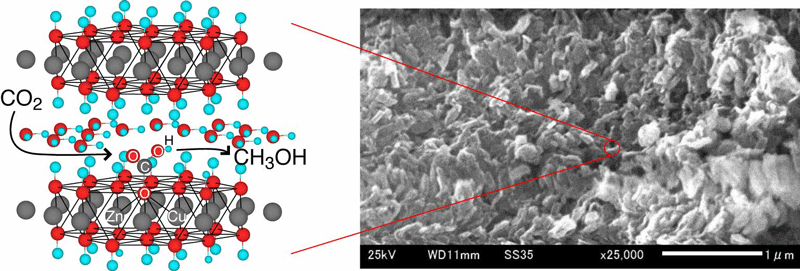
Ordered layered double hydroxides (LDHs) consisting of zinc and/or copper hydroxides were synthesized and combined with aluminum or gallium. These LDH compounds were then applied as photocatalysts to convert gaseous CO2 (2.3 kPa) to methanol or CO under UV-visible light by using hydrogen. Zn–Al LDH was the most active for the CO2 photoreduction and the major product was CO formed at the rate 620 nmol h**–1 g(cat)**–1, whereas methanol was the major product by the inclusion of Cu in the LDH photocatalysts, e.g. at the formation rate 170 nmol h**–1 g(cat)**–1 using Zn–Cu–Ga photocatalyst. The methanol selectivity improved from 5.9 to 26 mol% and 39 to 68 mol% by using Zn–Al (the conversion 0.16–0.11%) and Zn–Ga LDH catalysts (the conversion 0.02–0.03%), respectively, by the inclusion of Cu. Specific interaction of Cu sites with CO2 was spectroscopically suggested to enable coupling with protons and photogenerated electrons to form methanol.
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group