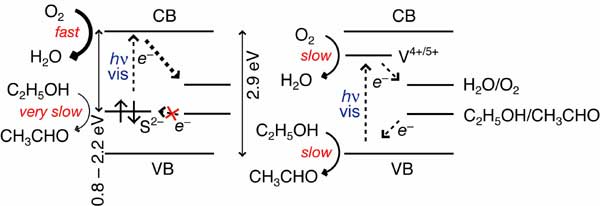
Anion doping to titanium oxide (TiO2) has been extensively studied to explore visible light-responsive photocatalysts. Among them, sulfur doping was most effective; however, the site structure and the implication to promoted photocatalysis have not been reported yet, except for valence state information based on X-ray photoelectron spectroscopy. Sulfur and/or nitrogen was doped to TiO2 with a narrow pore size distribution, at 3 nm, using thiourea or urea. They were compared to the uniform mesoporous TiO2 modified via the chemical vapor deposition (CVD) of hydrogen sulfide and the titanate/TiO2 nanotube synthesized hydrothermally using TiS2. In ethanol and O2 under visible light (wavelengths of >370, >420, >480, >520, or >580 nm), water formation was promoted by a factor of 12, in clear contrast to the only 2.3Å~ enhancement for acetaldehyde formation by doping sulfur. These anion-doped mesoporous TiO2 catalysts were characterized using Ti and S K-edge extended X-ray absorption fine structure for the first time. The Ti-S bonds were detected at 2.283-2.44 Angstrom for sulfur-doped mesoporous TiO2 using thiourea and sulfur-doped mesoporous TiO2 via CVD, demonstrating the presence of substitutional anionic S on the O sites. The doped anionic sulfur created impurity level(s) at 0.8-2.2 eV below the conduction band (CB) minimum of mesoporous TiO2. It enabled the electron excitation to the CB and then to O2 to convert to water under visible light. In contrast, the impurity level(s) at 0.8 - 2.2 eV below the CB minimum was/were not effective to (directly or indirectly) receive electrons from the substrate (ethanol/ethoxyl). Preferential water formation under visible light was also observed for TiO2 nanotubes that were synthesized hydrothermally from TiS2.