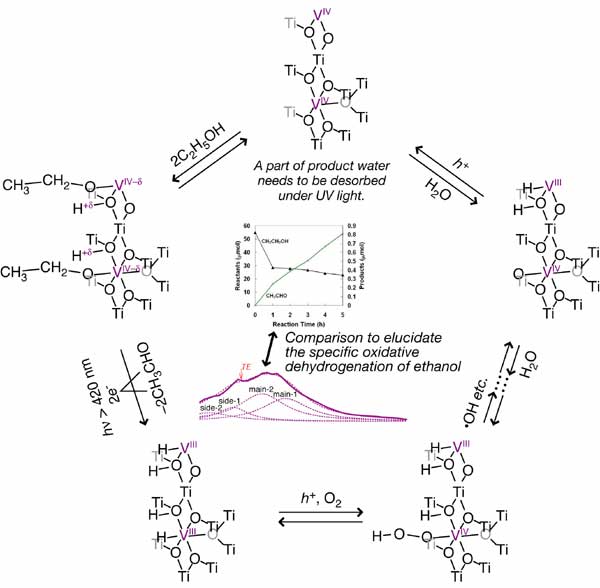
Selective oxidation under visible light is attractive for application to environment-benign societies. Oxidative dehydrogenation of ethanol proceeds over mesoporous V-doped TiO2 under visible light in clear contrast to ethanol dehydrogenation over crystalline TiO2 doped or undoped with V and inactive mesoporous TiO2. The red-ox states of V(IV) and V(III) were detected using X-ray spectroscopies. In this paper, kinetic measurements of each catalytic step and X-ray spectroscopic monitoring under catalytic reaction conditions are correlated. The ethanol conversion to acetaldehyde in the absence of O2 was monitored under visible light coupled with reduction of V(IV) to V(III) species based on V Kbeta5,2 emission and V Kbeta5,2-selecting X-ray absorption fine structure spectra. The oxidative dehydrogenation of dissociatively adsorbed ethoxyl species proceeded in O2 under visible light with formation rates 38% of steady photo-catalysis in ethanol + O2. The acetaldehyde desorption step by the H subtraction from ethoxyl species on V(III) was found to be rate-limiting. The origin of oxidative dehydrogenation over mesoporous V-TiO2 was suggested to be coordinatively unsaturated V(III) species specifically activating O2 molecules. Further, poisoning effect of product water was demonstrated to block the active V sites. UV light illumination was found to re-activate the mesoporous V-TiO2 catalyst.