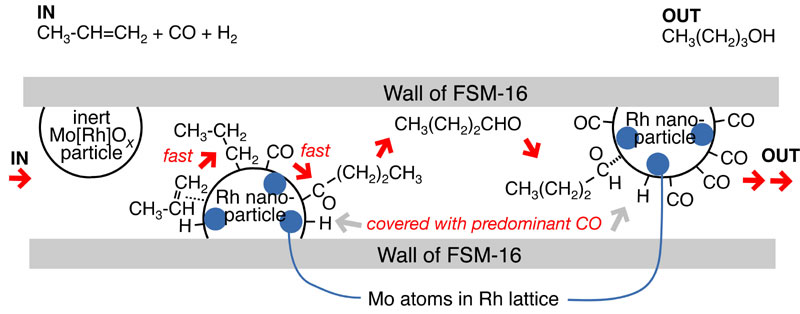
Rhodium catalysts were synthesized in ordered mesoporous silica FSM-16 using Rh/Al heteropolyacid anions and/or [Rh(COD)2]+ complex. The hydroformylation activity of propene was compared to impregnated Rh/FSM-16 catalysts prepared from Rh chloride. The catalysts were very selective to produce butanols due to the effect of 2-dimensional mesoporous reaction space (effective internal pore diameter 27 angstroms. The selectivity to butanols (n-butanol and i-butanol) was as much as >98% over [RhMo6O18(OH)6]3-/FSM-16 catalysts and 73% over RhCl3/FSM-16 catalysts. Under the hydroformylation reaction conditions at 433 K and 60 kPa, 22 - 28 angstroms of supported nanoparticles were present based on Rh K-edge EXAFS (extrended X-ray abosorption fine structure) and HR-TEM (high-resolution transmission electron microscopy) measurements. Rh metallic nanoparticles atomically mixed with Mo atoms and distorted heteropolyacid species coexisted in the [RhMo6O18(OH)6]3-/FSM-16 catalysts based on Rh and Mo K-edge EXAFS and HR-TEM measurements. The population of metallic nanoparticles increased as the metal loading amount decreased from 5.2 to 0.22 wt% Rh. Thus, metallic nanoparticles in FSM-16 were essential for the butanol synthesis and Mo played additional promoter role to increase the selectivity further. A reaction mechanism was proposed in which metallic nanoparticle surface was predominantly adsorbed with CO and multiple-step hydrogenations of oxygenate intermediates proceeded to form butanols during slow diffusion in the 2-dimensional mesoporous space.