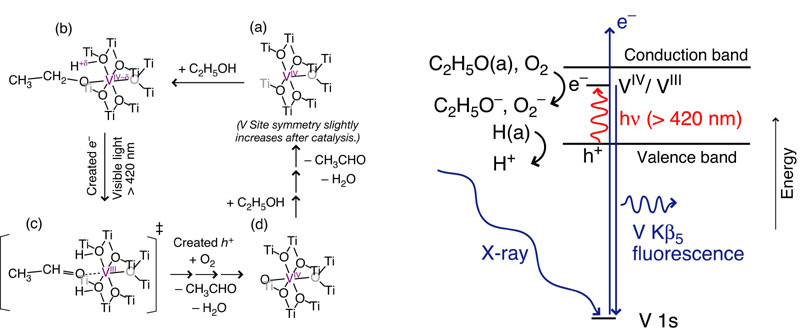
The photocatalytic role of vanadium doped in mesoporous TiO2 has not been clarified. Valence state-sensitive V K beta5,2-selecting (5462.9 eV) X-ray absorption fine structure (XAFS) was used to monitor the V sites in mesoporous TiO2 for ethanol dehydration under equilibrium in situ conditions and visible light-illumination. First, the feasibility of discriminating V(IV) sites from a 1:1 physical mixture of standard V(IV) and V(V) inorganic compounds was demonstrated, by tuning the secondary fluorescence spectrometer to 5459.0 eV. The chemical shift of V K beta5,2 emission between V(IV) and V(V) sites was 1.0 eV. The selection of valence states V(IV) and V(V) was 100% and 80%, respectively. The redox states for ethanol dehydration over mesoporous TiO2 excited in visible light were suggested to be V(III) and V(IV). The chemical shift between valence states V(III) and V(IV) was greater (3.2 eV). On the basis of V K beta5,2 emission and V K beta5,2-selecting XAFS spectra tuned to the V K beta5,2 peak, we determined that the fresh mesoporous V-TiO2 catalyst has a valence state of V(IV). The vanadium sites were partially reduced by the dissociative adsorption of ethanol under visible light, but they still stay within the emission-energy ranges for standard V(IV) compounds. These partially reduced vanadium sites were reoxidized in oxygen under visible light. Finally, direct XAFS observation of photoreduced V(III) sites was attempted by tuning the fluorescence spectrometer to 5456.3 eV for partially reduced mesoporous V-TiO2. Valence state V(III) was selected for 60% of the spectrum in the mixture of V(III) (minor) and V(IV) (dominant) valence states.