Characterization of Intercalated Iron(III) Nanoparticles and Oxidative Adsorption of Arsenite on Them Monitored by X-ray Absorption Fine Structure Combined with Fluorescence Spectrometry |
Yasuo Izumi, Dilshad Masih, Ken-ichi Aika, and Yoshimi Seida, Journal of Physical Chemistry B, 109(8), 3227 - 3232 (2005).
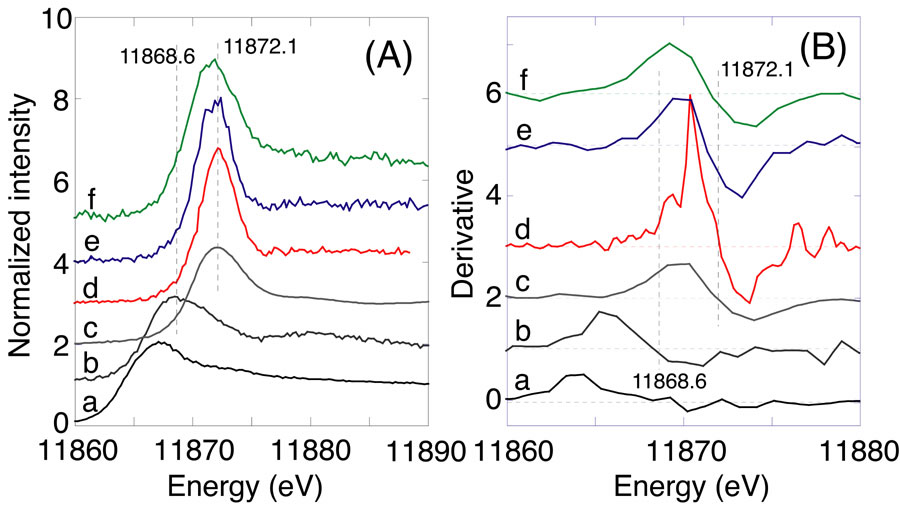
This paper first deals with the screening and optimization of FeIII-based adsorbents for arsenic adsorption from 0.2 - 16 ppm test solutions of arsenite/arsenate. Best adsorption capacity has been reported on alpha-FeO(OH) on adsorbent weight basis. Better results were found on intercalated Fe-montmorillonites for arsenite adsorption below the equilibrium dissolved As concentration of 310 ppb and for arsenate adsorption in all the concentrations studied. Next, the speciation of As adsorbed was performed by As K-edge XAFS combined with high-energy-resolution fluorescence spectrometry. Major oxidative adsorption of arsenite was observed on Fe-montmorillonite from the 0.2 - 16 ppm test solutions. The reasons of higher capacity of arsenic adsorption and oxidative adsorption of arsenite on Fe-montmorillonite are discussed.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group