X-ray Absorption Fine Structure Combined with X-ray Fluorescence Spectrometry. Improvement of Spectral Resolution at the Absorption Edges of 9 - 29 keV |
Yasuo Izumi, Hiroyasu Nagamori, Fumitaka Kiyotaki, Dilshad Masih,
Taketoshi Minato, Eric Roisin, Jean-Pierre Candy, Hajime Tanida, Tomoya Uruga
Analytical Chemistry, 77(21), 6969 - 6975 (2005).
[PDF file]
Analytical Chemistry, 78(7), 2075 (2006). [PDF file of a correction]
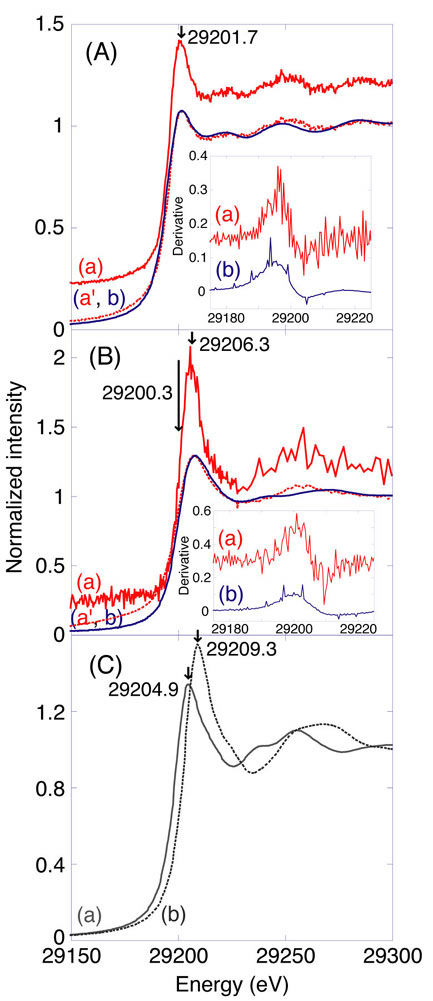
X-ray absorption near-edge structure (XANES) suffers from core-hole lifetime broadening at higher-energy absorption edge, such as Sn K (29 keV, core hole lifetime width for K = 8.49 eV). To overcome this problem, emitted Sn Kalpha1 fluorescence from sample was counted using high-energy-resolution fluorescence spectrometer in the XANES measurements. Experimental energy resolution (5.0 eV) was consistent with theoretical values based on the Rowland configuration of the spectrometer. The absorption edge became steeper compared to conventional spectra. The white-line peak due to Sn(II) species became remarkably sharper and more intense in the Sn Kalpha1-detecting Sn K-edge XANES for Pt-Sn/SiO2. To support the semi-classical theory of resonant Raman scattering for the explanation of observed elimination of lifetime width, more resolved XANES data at Cu K, Pb L3, and Sn K in this work were convoluted (filtered) with a Lorentzian of each core-hole lifetime width. The processed data resembled generally well corresponding XANES spectrum measured in transmission mode. The verification based on ab initio XANES calculations was also performed.

Normalized Sn K-edge XANES spectra for Sn metal (A) and Pt-Sn/SiO2
catalyst (2.5 wt%-Pt; Sn/Pt atomic ratio = 1.0) (B) measured by detecting Sn
Kƒ¿1 fluorescence (a) or in transmission mode (b). The data a were convoluted
by a Lorentzian of core-hole lifetime width for Sn K level (8.49 eV) to be a'
(dotted line). (Inset) First derivative spectra of normalized XANES (a and b).
(C) Sn K-edge XANES for SnO (a) and SnO2 (b) measured in transmission mode.
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group