Nitric Oxide Reduction by Carbon Monoxide over Supported Hexaruthenium Cluster Catalysts. 1. The Active Site Structure That Depends on Supporting Metal Oxide and Catalytic Reaction Conditions |
Taketoshi Minato, Yasuo Izumi, Ken-ichi Aika, Atsushi Ishiguro,
Takayuki Nakajima, and Yasuo Wakatsuki
Journal of Physical Chemistry, B, 9022 - 9028, 107(34) (2003).
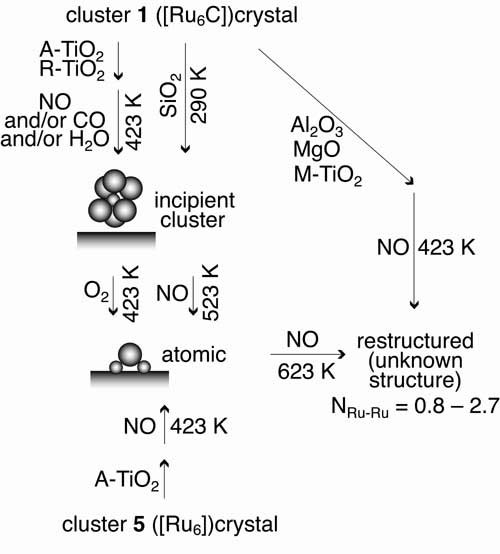
Ruthenium site structures supported on metal oxide surfaces were designed by reacting organometallic Ru cluster [Ru6C(CO)16]2- or [Ru6(CO)18]2- with various metal oxides, TiO2, Al2O3, MgO, and SiO2. The surface Ru site structure, formed under various catalyst preparation and reaction conditions, was investigated by the Ru K-edge extended X-ray absorption fine structure (EXAFS). Samples of [Ru6C(CO)16]2-/TiO2(anatase) and [Ru6C(CO)16]2-/TiO2(rutile) were found to retain the original Ru6C framework when heated in the presence of NO (2.0 kPa) or NO (2.0 kPa) + CO (2.0 kPa) at 423 K, i.e. catalytic reaction conditions for NO decomposition. At 523 K, the Ru-Ru bonds of Ru6C framework were cleaved by the attack of NO. In contrast, the Ru site became spontaneously dispersed over TiO2 (anatase). When being supported over TiO2 (mesoporous), MgO, or Al2O3, the Ru6C framework split into fragments in gaseous NO or NO + CO even at 423 K. The Ru6 framework of [Ru6(CO)18]2- was found to break easily into smaller ensembles in the presence of NO and/or CO at 423 K on support. Taking into consideration the realistic environments in which these catalysts will be used, we also examined the effect of water and oxygen. When water was introduced to the sample [Ru6C(CO)16]2-/TiO2(anatase) at 423 K , it did not have any effects on the stabilized Ru6C framework structure. In the presence of oxygen gas, however, the Ru hexanuclear structure decomposed into isolated Ru cations bound to surface oxygen atoms of TiO2 (anatase).

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group