Atsushi Ishiguro, Takayuki Nakajima, Tadahisa Iwata, Masahiro Fujita, Taketoshi Minato, Fumitaka Kiyotaki, Yasuo Izumi, Ken-ichi Aika, Masaya Uchida, Koji Kimoto, Yoshio Matsui, Yasuo Wakatsuki
Chem. Eur. J., 3260-3268, 8(14) (2002).
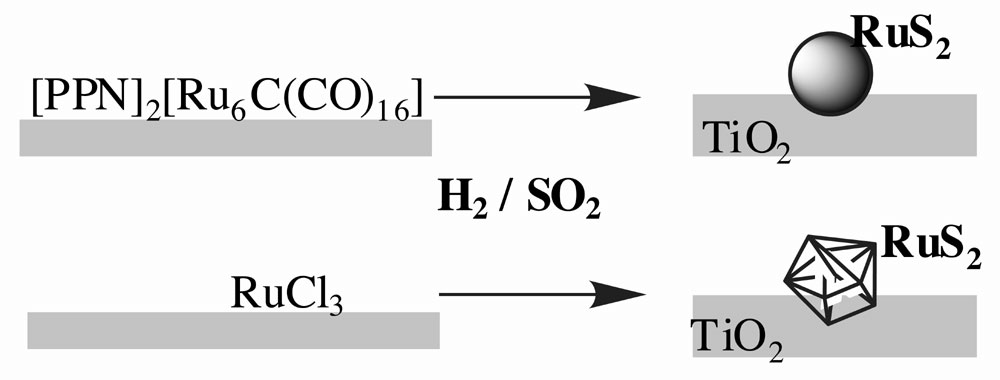
TiO2-supported ruthenium metal-particles were derived from an anionic hexanuclear carbido carbonyl cluster [Ru6C(CO)16](2-) and compared with those prepared conventionally via impregnation of TiO2 with a solution of RuCl3 followed by reduction with H2. The average sizes of the metal particles in both systems were similar, i.e. 12 angstroms for molecular cluster-derived particles and 15 angstroms for those derived from the RuCl3-precursor, although the size-distribution was sharper in the former case. These supported particles efficiently promoted the reduction of SO2 with H2 to give elemental sulfur. Their active form was ruthenium sulfide as confirmed by EXAFS and X-ray diffraction measurements. The nano-scale ruthenium sulfide particles, which originated from the cluster complex, had an amorphous character and showed activity even at low temperature (463 K), whereas ruthenium sulfide formed via RuCl3-derived metal dispersion was pyrite-type RuS2 crystallite and needed a temperature above 513 K to effect the same catalysis. Amorphous ruthenium sulfide maintains its nano-sized scale (~14 angstroms regardless of the reaction temperature, while RuS2 crystallite aggregates to form larger non-uniform particles.
Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group