Ethanol Synthesis from Carbon Dioxide on [Rh10Se]/TiO2 Catalyst Characterized by X-Ray Absorption Fine Structure Spectroscopy |
Y. Izumi, H. Kurakata, and K. Aika,
J. Catal., 236 - 244, 175 (1998).
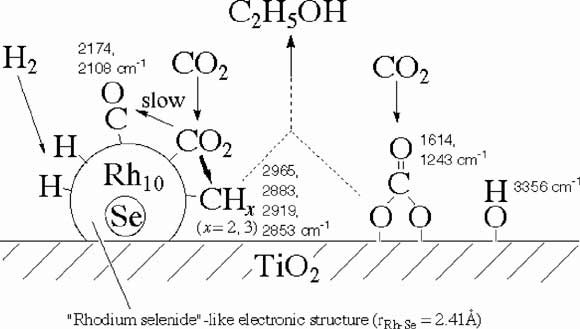
The active site structures and electronic states were studied for the [Rh10Se]/TiO2 catalyst by means of edge spectra and EXAFS. The rate and selectivity of ethanol synthesis from CO2 on [Rh10Se]/TiO2 had strong dependence on the heating temperature in vacuum (T(evac)). Corresponding to the maximum of rate and selectivity when the T(evac) was 623 K, the distance r(Se-Rh) reached a minimum (0.241 nm) by Se K- and Rh K-edge EXAFS analyses. The contracted [Rh10Se] cluster was found to have an electronic state similar to that of Rh3Se8 or RhSe2 rather than of metallic Rh. A new reaction path control is proposed by regulating the distance between the interstitial Se atom and the metal framework [Rh10]. A strong peak due to 1s -> np transition was observed around the Se K absorption edge. The peak intensity did not exhibit significant change when the T(evac) was varied for [Rh10Se]/TiO2. On the other hand, the area of peak observed around 23,230 eV in Rh K-edge spectra gradually decreased when the T(evac) was elevated for [Rh10Se]/TiO2. Hence, the Se atom surrounded by [Rh10] framework was always kept in anionic state, while the electronic state of Rh atoms gradually changed by the interaction with TiO2 surface. The change for Rh atoms was also supported by the gradual increase of r(Rh-Rh) obtained by EXAFS with the temperature increase. In the case of Rh sites with lower total coordination number around Rh ([Rh10Se]/SiO2, [Rh10Se]/Al2O3, and [Rh10Se]/MgO), the reaction CO2 -> CO(a) + O(a) occurred predominantly and formed CO(a) poisoned the catalysis.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group