Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
Catalysis on Ruthenium Clusters Supported on CeO2 or Ni-doped CeO2: Adsorption Behavior of H2 and Ammonia Synthesis |
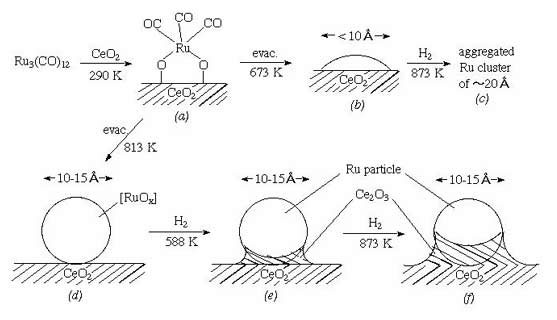
Catalysis on Ru clusters supported on CeO2 or Ni-doped CeO2 was investigated. The Ru3(CO)12 was reacted with CeO2, followed by heating in vacuum at 673 or 813 K, then in H2 at 588 - 1073 K (T(H2)). Ammonia synthesis activities of both catalysts had the T(H2) dependence, which has a maximum at T(H2) = 873 K. On a sample in which Ru3(CO)12 was supported on previously reduced Ni/CeO2, the highest synthesis rate was 1.5Å~10**-3 mol h-1g•cat(-1) at T(H2) = 588 K. The activity order can be understood in terms of two factors: (A) reduction extent of support, and (B) number of active Ru sites. The two factors conflicted with each other when the treatment temperature in H2 increased. By heating the samples in H2 up to 873 K to satisfy factor (A), the aggregation of Ru clusters or physical blocking of surface Ru sites by CeO(2-x) occurred: factor (B) was not satisfied. The two factors should be optimized in catalyst Ru3-Ni/CeO2, where the support cerium oxide was thoroughly reduced through the doped Ni. On reduced Ni/CeO2, the Ru cluster implantation can be done at low temperature (588 K). Obtained values of r(Ru-Ru) at 0.262 nm (N = 7.1) and r(Ru-O(s) )(O(s): oxygen atom at surface) at 0.212 nm (N = 1.2) by EXAFS for Ru3-Ni/CeO2 suggested a flat Ru cluster model comprised of several Ru atoms on reduced Ni/CeO(2-x) surface. The H(a)/Ru(total) ratio exceeded unity, suggesting new H adsorption sites. The temperature programmed desorption for hydrogen (simultaneous desorption of HD and D2 at 330 - 430 K) suggested that the H at the new site and H on Ru surface were exchangeable above 330 K. The "reservoir" effect of the new site for H on catalysis is discussed in relation to new kinetic design of hydrogenation catalyst.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group