Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
Promoted Catalysis by Supported [Ru6N] Clusters in N2 and/or H2: Structural and Chemical Controls |
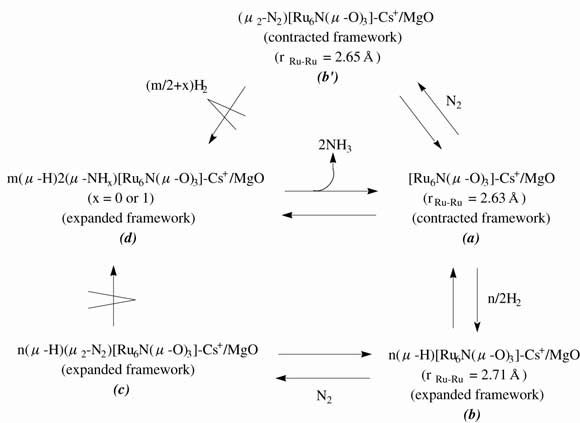
Ammonia synthesis on supported nitrido clusters [Ru6N] was investigated as a potential catalysis system of transition metal + main group element in relation to the framework structure (change) of [Ru6N] clusters in H2 or N2 as found in the accompanying paper. The [Ru6N(ƒÊ-O(s))3] (O(s): oxygen atom at surface) clusters were prepared from the [Ru6N(CO)16](-) cluster on MgO, K(+)-doped MgO, and Cs(+)-doped MgO, and the stability in reaction conditions of ammonia synthesis was probed by EXAFS. The reaction rates on these nitrido clusters were found to be faster than non-nitrido [Ru6] cluster prepared from [Ru6(CO)18](2-), degraded [Ru3(ƒÊ2-O(s))3] cluster or aggregated Ru clusters (N(Ru-Ru) = 6.2 - 6.6) prepared from [Ru6N(CO)16](-), or conventional Ru catalysts. Also, the H2-D2 exchange reactions (in the presence/absence of N2) proceeded faster on supported [Ru6N] clusters than the other catalysts. The Ru wt% dependence of ammonia synthesis activities on [Ru6N]/MgO suggested the importance of Ru-hexamer ensemble and cluster/support interface for the catalysis. Related to the coordination structures of H or N2 and structure changes of [Ru6N] framework in H2 or N2 in the accompanying paper, the promoted reaction mechanism of ammonia synthesis on supported [Ru6N] clusters is discussed in terms of (1)nuclearity of Ru, (2)cluster/support interface, (3)structural effect through expansion/contraction of the [Ru6N] framework, and (4)electron donation by nitrido nitrogen, based on in-situ EXAFS, in-situ IR, H2-D2 exchange reactions, reaction orders, and H/D isotope effects.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group