Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
Preparation of [Ru6N] Clusters on MgO, K+/MgO, Cs+/MgO, and Al2O3 and the Reactivities with H2 and N2 |
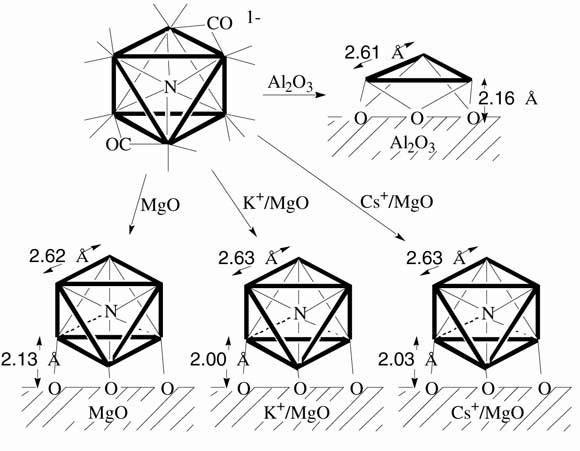
The supported ruthenium clusters [Ru6N] were prepared on MgO, K(+)/MgO, and Cs(+)/MgO from [Ru6N(CO)16](-) cluster as hydrogenation catalysts stabilized and chemically-modified by nitrido nitrogen. The characterization and reactivities with H2 and N2 were investigated by EXAFS in relation to their catalysis promoted by nitrido nitrogen. The [Ru6N] cluster unit was found to remain on MgO after heating in vacuum at 813 K (decarbonylated) and in H2 at 588 K (reaction condition of N2 hydrogenation), whereas the [Ru6N(CO)16](-) cluster strongly interacted with the Al2O3 surface to degrade to [Ru3] by heating in vacuum at 813 K. The decarbonylated [Ru6N] framework also remained in H2 at 588 K on K(+)/MgO and Cs(+)/MgO without aggregation nor degradation. By changing the Ru loading from 0.48 to 3.9 wt% on MgO, the coordination number N(Ru-O(s)) (O(s): oxygen atom at surface) decreased from 1.2 to 0.3, while the [Ru6N] cluster unit remained unchanged for samples with Ru loading up to ~2.5 wt%. The preferable reaction of [Ru6N] cluster with MgO(001) flat surfaces was suggested for the sample at Ru 2.5 wt%, but the cluster should have reacted mainly with lower-coordination sites of MgO for the sample with lower Ru loading (~0.5 wt%). The r(Ru-O(s))was shorter for [Ru6N] on K(+)/MgO and Cs+/MgO (2.00 - 2.03ü) than on MgO (2.13ü), implying the [Ru6N] was interacted with O(s) atoms bonded to K(+) or Cs(+) ions to have direct support effect of basic oxide K(+)/MgO or Cs(+)/MgO on catalysis. H-induced structure changes were observed for [Ru6N]/MgO and [Ru6N]-Cs(+)/MgO as the reversible changes of bonding distance r(Ru-Ru) of 0.03 and 0.08ü, respectively, by the adsorption/desorption of H2. The adsorption of N2 was simple adsorption on [Ru6N] without structural change of [Ru6N] on MgO or Cs(+)/MgO.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group