Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
CO-Breathing Structure Change and Catalysis for Oxygenate Synthesis from CO/H2 on Supported [Ru6C] Clusters: Structural and Chemical Controls by Interstitial Carbido Carbon |
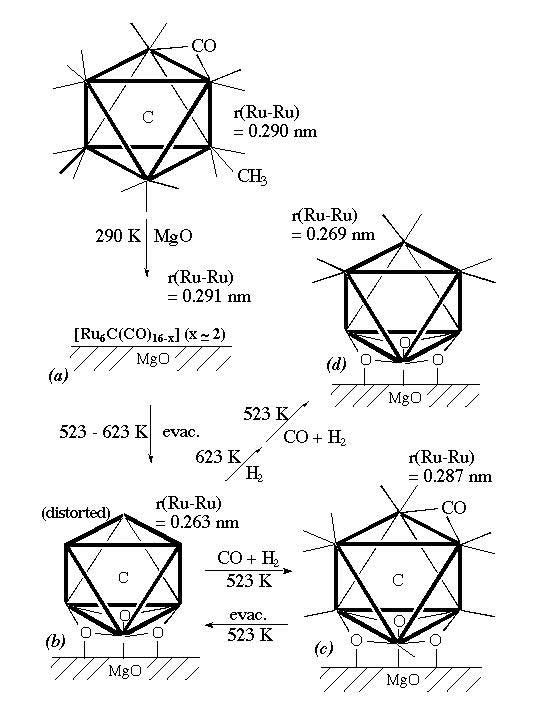
Catalysis and structures of supported ruthenium carbido clusters [Ru6C(CO)16(CH3)](-)/oxide were investigated in comparison with those of supported non-carbido clusters prepared from [Ru6(CO)18](2)- and traditional ruthenium catalysts prepared from Ru salt. Oxygenate synthesis (methanol, dimethyl ether, and formaldehyde) in CO-H2 reaction was observed on the supported carbido clusters in contrast to the preferential formation of methane and hydrocarbons on the conventional Ru catalysts and the supported non-carbido clusters. The active structure for oxygenate synthesis crucially depended on the kind of support oxides. On basic oxides (MgO and La2O3), the cluster of framework was incorporated with surface oxygen atoms and expanded or shrunk under CO-H2 reaction conditions depending on the CO pressure, as demonstrated by EXAFS. The reversible expansion - shrink of the cluster framework under CO-H2 was correlated with the activated CO adsorption ("CO breathing"-induced structural change). On TiO2, the [Ru6C] framework always held a shrunk structure. The expanded clusters showed high selectivities in oxygenate synthesis. Infrared spectroscopy and hydrogen isotope effects suggested the formation of oxygenates through a mu(2)-formyl intermediate. The switchover of reaction path from the formation of methane and hydrocarbons to oxygenate synthesis is ascribed to the interstitial carbido carbon. It has a structural effect like a central spring and electronic effect as a four-electron donor on the behavior of the cluster framework.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group