Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
Selective Carbonyl Insertion and Ethene Hydroformylation on a [Ru6C(CO)16Me](-)-SiO2 Catalyst |
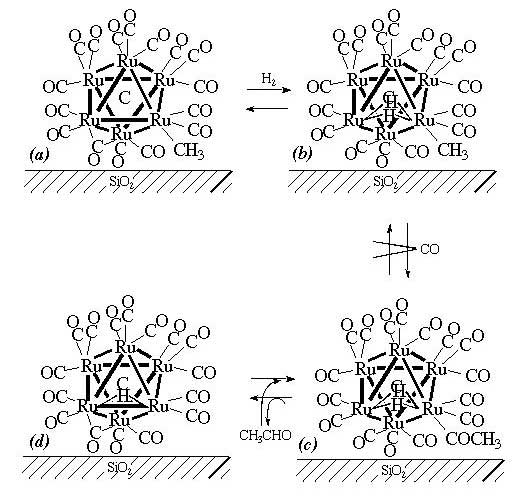
Stoichiometric acetaldehyde formation by insertion of CO into the methyl ligand and catalytic ethene hydroformylation on the cluster, [Ru6C(CO)16Me](-) supported on SiO2 at 373 - 473 K have been investigated to understand the effects of the catalysis on the metal cluster framework and also to develop new catalytic systems on a molecular scale. Two elementary steps for stoichiometric acetaldehyde formation, (1) methyl -> acetyl and (2) acetyl -> acetaldehyde, were observed by FT-IR. The rate of (1) in CO + H2 was faster than that in CO, suggesting a hydride-promoted mechanism of carbonyl insertion. The hydride promotion and hydrogen gas pressure dependence suggested dissociative adsorption of H2 so as to bridge a Ru-Ru bond and the incorporation of the multi-Ru sites in the acetaldehyde formation mechanism. The reductive elimination of hydride and methyl ligands upon methane formation was much slower than the reductive elimination of H and MeO for acetaldehyde formation as well as the insertion of CO (methyl migration) for acetyl formation. In terms of this specific feature, the catalytic hydroformylation of ethene was found to proceed on the catalyst with nearly 100% selectivity at 398 K in the case of highly dehydrated SiO2. The retention of the cluster framework under the reaction conditions was confirmed by EXAFS. On the contrary, [Ru6C(CO)16Me](-) in a homogeneous system did not catalyze this reaction and conventional impregnation Ru/SiO2 catalysts showed 0 - 0.09% selectivities. A reaction mechanism is presented.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group