Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group
Promoting Effects of Se on Rh/ZrO2 Catalysis for Ethene Hydroformylation |
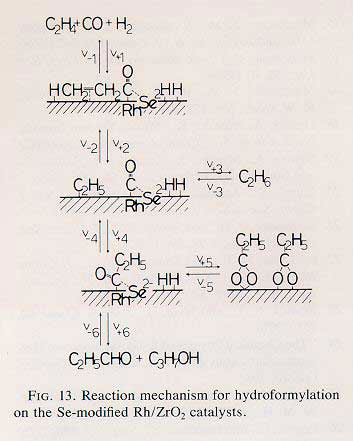
The positive effects of selenium added to Rh/ZrO2 catalysts in ethene hydroformylation were studied from the viewpoints of promoter effects of electronegative atoms, normaly regarded as poisoning atoms as well as electronic interactions with both substrate metal and adsorbed species. Se-modified Rh/ZrO2 catalysts prepared by the reactive deposition of Me2Se or co-impregnation method using SeO2 were found to be active and selective for ethene hydroformylation. The addition of selenium promoted the formation of propanol rather than propanal, the propanol formation being increased more than 70 times at an optimum Se : Rh ratio. The propanoyl (MeCHO-) intermediates were observed at ca. 1770 cm(-1) and converted reversibly to propionate (MeCO2-) species (bridge and bidentate) observed at 1425 - 1575 cm(-1). The ensemble of Se(2-)/Rh was suggested to promote CO adsorption and the insertion of CO to ethyl species, where there is electronic interaction between Se(2-) and CO or propanoyl species. The isotope effects of D2 and (13)C(18)O showed the switchover of the rate-limiting step for hydroformylation from the CO insertion on the Rh/ZrO2 catalysts to the dissociative adsorption of H2 on the Se-Rh/ZrO2 catalysts. The role of Se is discussed in relation to the reaction mechanism of oxygenate formations.

Chiba University > Graduate School of Science > Department of Chemistry > Dr. Yasuo Izumi Group